Crystallography Sample Supports System
Crystallography Sample Supports will be a next-generation crystal mounting system that changes how crystals can be prepared for X-ray diffraction data collection.
This new system allows users to –
- Mount single crystals or thousands for micro-crystals
- Optimize for serial crystallography or single crystal XRD
- Use for cryogenic or room temperature diffraction
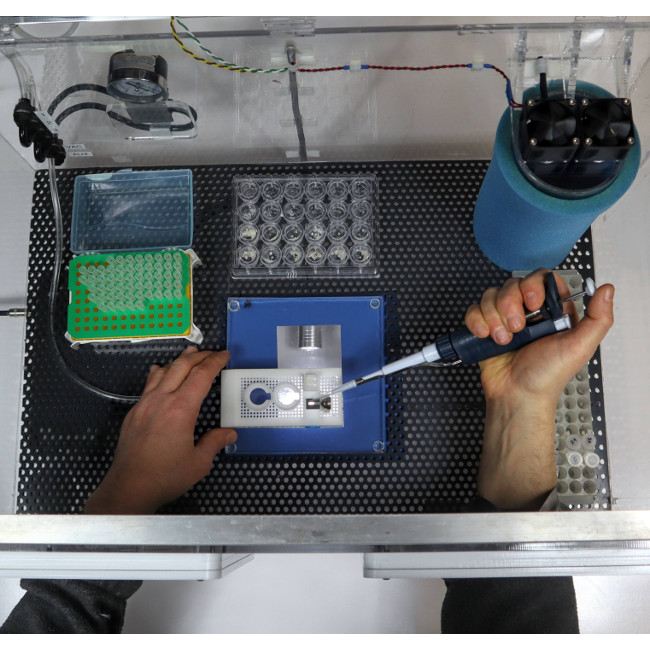
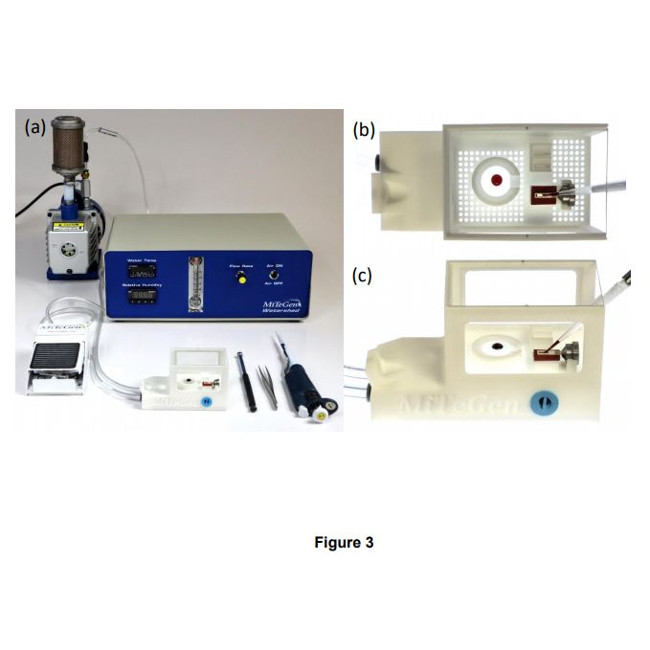
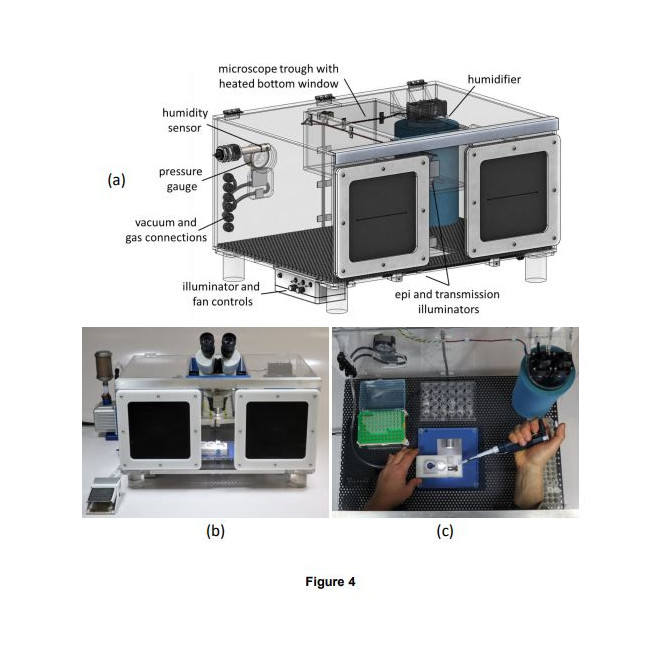
How it works – Crystals are mounted onto the supports utilizing a humidified sample loading box. The high-humidity box allows samples to be prepared without unintentional dehydration to the drops or crystals. The supports can than be plunge cooled (for cryo) or sealed (for room temperature work).
The system has multiple applications –
- Conventional Crystallography
- Serial Crystallography
- In Situ Crystallography
- Room Temperature Crystallography
- And more
Patent Pending
Learn More About the Crystallography Sample Supports System
OR
Product Information
The Crystallography Sample Support system is an integrated system for preparing samples for several types of crystallography research. This system integrates and improves upon previously demonstrated concepts to deliver a versatile and high-performance solution.
The system's several salient features are:
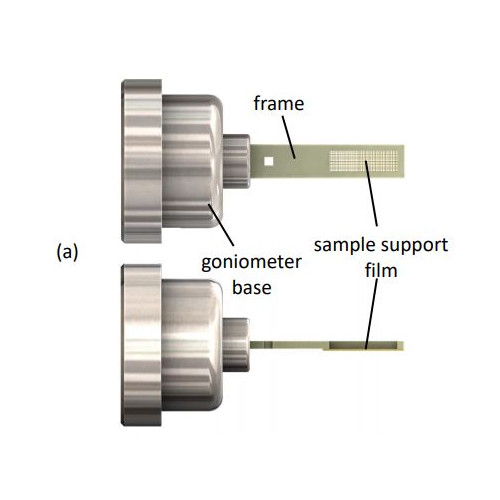
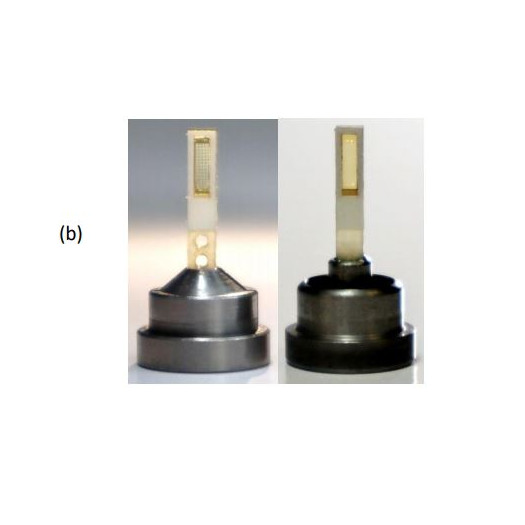
- The sample supports are compatible with existing infrastructure for home source and mail-in SR crystallography, including sample handling tools, storage cassettes/pucks, automounters, and goniometer stages. This reduces overall cost and increases opportunities for data collection.
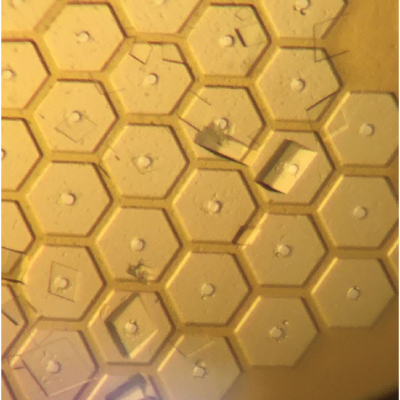
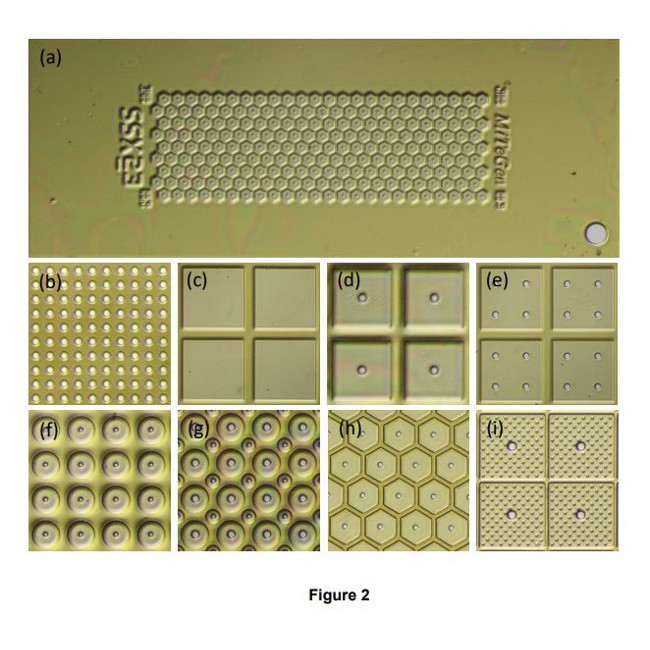
- A single sample holder platform accepts diverse sample support film designs for different size/shape crystals that aid in achieving regular or dispersed crystal positioning.
- Imaging of microcrystals as small as 1-2 µm over the entire active area of the support – not just in wells - is straightforward using both standard visible light microscopy and laser scanned nonlinear excitation modalities due the very thin polymer windows in each well, the thin polymer walls between wells, easy removal of crystal-contrast reducing surrounding liquid, and polyimide’s weak TPEF and zero SHG signals.
- With one or two stages of humidity control provided by the humidity-controlled sample loading station and the humidified glovebox (SLEEC), dehydration of crystallization drops and crystals – a critical issue when loading microcrystals for room temperature data collection – can be eliminated. As-grown crystal isomorphism is maintained, scaling and merging of diffraction data from many crystals is improved, and the number of crystals required for structure determination is reduced.
- Foot-pedal modulated suction within a humidified environment gives effective control of solution and crystal flows needed to achieve desired crystal positioning (e.g., random dispersion or at regularly spaced holes.) Positioning over holes can also be achieved by growing crystals in situ using vapor diffusion.
- Crystal position control is achieved using only thin polyimide films with micropatterned features only ~5-20 µm tall. Wells/walls defined by thick (130-250 µm) substrates (e.g., silicon, polycarbonate) are not necessary. Background X-ray scatter is low at all positions on the sample support. Diffraction data can be collected from essentially all crystals on the support, not just those located over holes or windows, increasing efficiency of crystal use. X-rays can be incident from and diffracted through a wide angular range without encountering the support frame. Large sample support rotations eliminate data collection issues caused by preferential crystal orientation, and complete data sets can be collected from suitably large individual crystals.
- For room temperature data collection, ~4 µm Mylar sealing films provide adequate protection against dehydration for data collection on a timescale of ~1-2 hours. For room temperature storage and shipping, sealed samples can be stored with reservoir solution in vials, Eppendorf tubes, and multiwell plates.
- Crystal cryoprotection is simplified and made more effective. Cryoprotectantcontaining solutions (or oils) can be deposited on crystals on the sample support, and then withdrawn using suction through the support's holes; repeating this deposition and removal two or more times can efficiently remove all solvent initially present on the crystal surface, eliminating the primary source of ice diffraction in cryocrystallography (Parkhurst et al., 2017; Moreau et al., 2020), and minimizing background scatter from excess cryoprotectant.
- For cryogenic temperature data collection, samples can be directly plunged in liquid nitrogen, and stored, shipped and handled in the same way and using the same tools as standard loops in cryocrystallography. IUCrJ BIOLOGY | MEDICINE research papers 24
- The supporting polyimide film is much thinner and has much less thermal mass per unit area than standard ~25 µm thick cryoEM grids. If ~1-2 µm crystals are used and excess solution carefully removed, cooling rates well in excess of 50,000 K/s (Kriminski et al., 2003; Warkentin et al., 2006), within a factor of ~10 of those achieved in typical cryoEM practice (>250,000 K/s), should be achievable using current state-of-the art LN2-based cooling methods. Consequently, capture via thermal quenching of roomtemperature biomolecular conformations should be nearly as effective using protein microcrystals as when using protein solutions on cryoEM grids (Kaledhonkar et al., 2019).
Patent Pending